Abstract
Introduction: Venetoclax is an oral, small-molecule BCL-2 inhibitor capable of restoring apoptosis in susceptible cancers. It is approved in the US for treatment of relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) with 17p deletion and in Europe for patients who are unsuitable for or who have failed B-cell receptor inhibitor (BCRi) therapy. It is also being evaluated in the broader CLL population and other hematologic malignancies. These diseases mostly afflict older patients, many of whom are treated with statins for high cholesterol. Aside from their cholesterol-lowering utility, statins have shown potential to induce apoptosis in various cancer cell lines, with evidence of synergism when combined with BCL-2 inhibition. On this background, post-hoc analyses of clinical trials in patients with CLL and multiple myeloma (MM) were undertaken to determine if statins have the potential to enhance the activity of venetoclax.
Methods: Data from CLL subjects were pooled from 3 single-arm, open-label Phase 1 or 2 trials of venetoclax monotherapy in subjects with R/R disease: Study 1 (NCT01889186) enrolled subjects exclusively with 17p(del) CLL; Study 2 (NCT01328626) was a first-in-human, dose-escalation study in subjects with CLL/SLL and NHL; Study 3 (NCT02141282) enrolled CLL subjects previously treated with ibrutinib or idelalisib. MM data were from 2 single-arm, open-label Phase 1 studies in patients with R/R disease: Study 4 (NCT01794507) evaluated venetoclax in combination with bortezomib and dexamethasone (VenVd); Study 5 (NCT01794520) evaluated treatment with venetoclax monotherapy (Ven) or venetoclax plus dexamethasone (Vendex). Previously, it was reported that single-agent activity of venetoclax is almost entirely restricted to t(11;14) positive MM; therefore, analyses of response among subjects who received Ven or Vendex were limited to this subgroup. The following CLL endpoints were analyzed based on investigator assessment: overall remission rate (ORR), the composite complete remission (CR) rate (CR + CR with incomplete marrow recovery), progression free survival (PFS), and overall survival (OS). Odds ratios for ORR and CR were compared via Fisher exact test. PFS and OS were analyzed by Kaplan-Meier methodology. Multivariate analyses were conducted using Cox proportional hazard model (OS and PFS) and logistic regression model (ORR and CR) with implementation of a stepwise model selection procedure. For the MM studies, descriptive statistics were prepared based on investigator assessment for ORR, partial response (PR), and very good partial response (VGPR) or better. Exposure-response analyses have previously shown that higher exposures to venetoclax in CLL patients are associated with greater likelihood of CR and longer PFS; therefore, we also investigated the potential for statins to increase venetoclax exposures utilizing steady state pharmacokinetic sampling from Study 2.
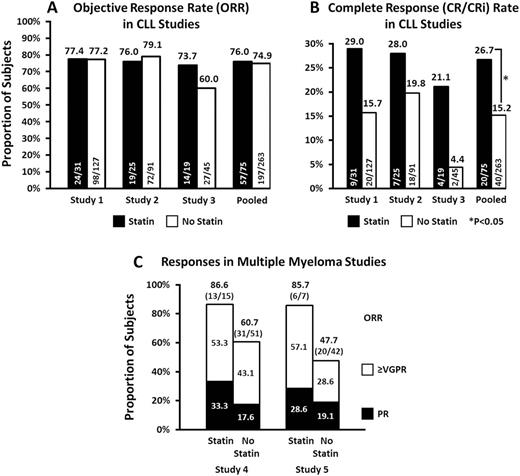
Results: In CLL studies, 22% of patients received a statin; statin users were more often male (79% vs. 67%), ≥65 years of age (73% vs. 54%), and enrolled at US sites (69% vs 46%). While the proportion of CLL subjects with any response (ORR) was similar for subjects with and without statin use (76% vs 75%), more statin users achieved CR, with cumulative CR rates of 26.7% vs. 15.2% in the pooled analysis. The unadjusted odds ratio (95% CI) for CR was 2.03 (1.099, 3.741; P=0.03) in favor of statin users, and the corresponding multivariable-adjusted odds ratio was 2.676 (1.338, 5.351; P<0.01). PFS and OS results were consistent with CR rates, with respective multivariable-adjusted hazard ratios of 0.61 (0.383, 0.956; P=0.03) and 0.50 (0.247, 1.007; P=0.05). The pharmacokinetic analysis showed no difference in venetoclax exposures between patients taking a statin versus those who did not. There were no unexpected safety observations in either group. The trend for improved response among statin-treated subjects was also present for the broad MM subject population treated with VenVd in Study 4 and t(11;14) positive MM subjects treated with Ven or Vendex in Study 5.
Conclusions: Statins were associated with enhanced response to venetoclax in retrospective analyses of CLL and MM clinical trials. Given the post-hoc nature of these findings and lack of randomization, further evaluation in a prospective, randomized clinical trial is required to draw definitive conclusions.
Roberts: AbbVie: Employment, Equity Ownership. Xu: AbbVie: Employment, Equity Ownership. Jia: AbbVie: Employment, Equity Ownership. Agarwal: AbbVie: Employment, Equity Ownership. Salem: AbbVie: Employment, Equity Ownership. Bellin: Abbvie: Employment, Equity Ownership. Lam: AbbVie: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal